
Real-time, remote data access.
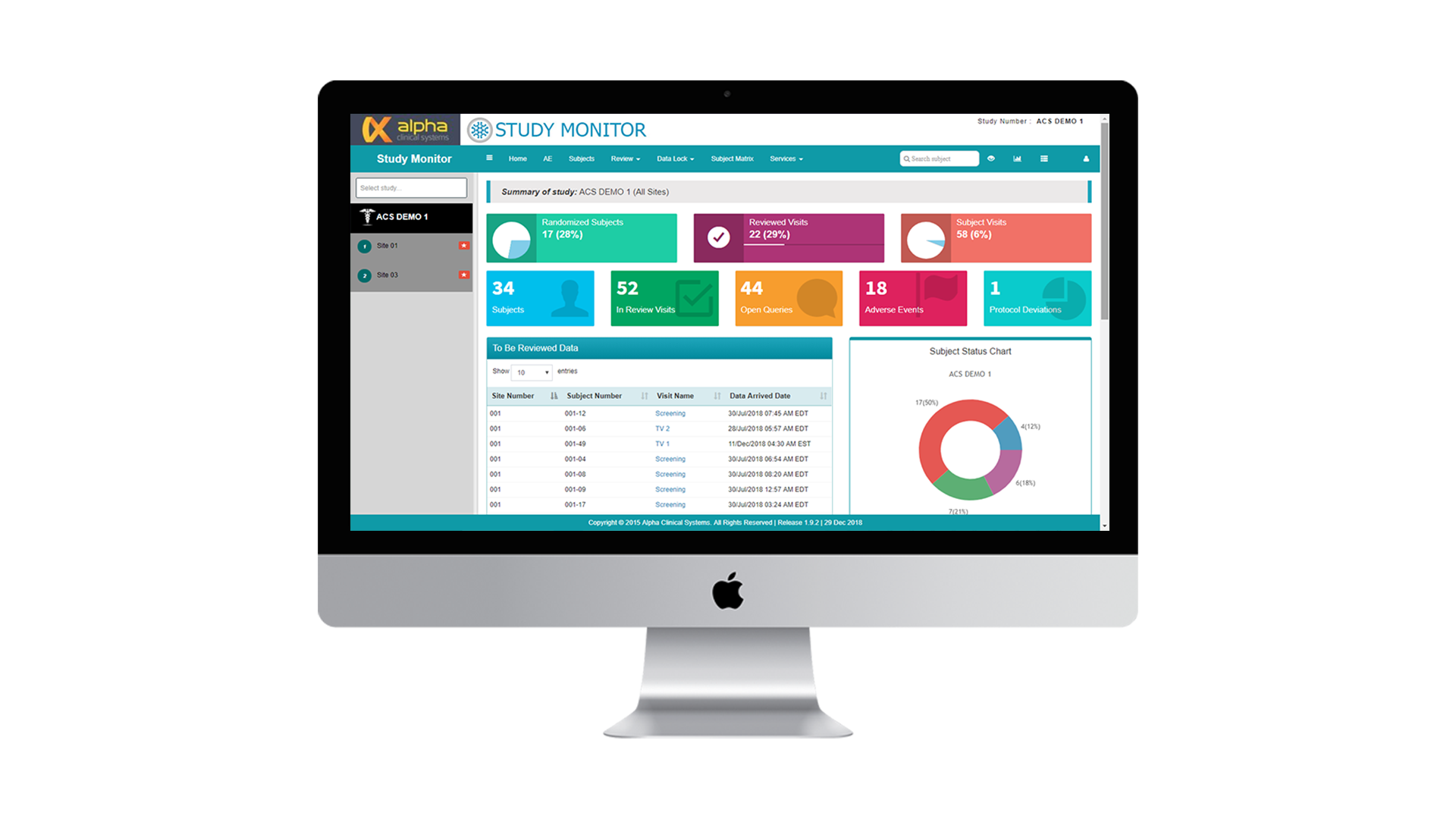
Maximize study transparency, safety and efficiency with real-time, remote access to eSource and eCRF data. Study Monitor seamlessly integrates with any ODM capable EDC system to optimize with eSource data capture.
Study Monitor
Real-time data for faster, safer,more innovative studies.
BENEFITS
- Facilitate remote, risk-based monitoring. Access study data remotely and in real time. From eSource and eConsent to drug inventory supply, wearable data and eHR data. View adverse events,protocol deviations and patient visit status in real time. User-friendly dashboards simplify remote monitoring with quick and easy query management.
- Reduce study timelines.Speed study timelines and reduce costs with fewer on-site monitoring visits. Improve patient care with a holistic view of patient data to identifying safety and efficacy signals and trends in a timely manner while making more informed dosing adjustments.
- Maximize compliance, minimize risk. Accelerated data access enables improved focus of resources and activities on areas of risk. Only direct eSource data capture delivers real time data access to prevent and mitigate risks to patient safety.
